At its bare and delicious bones, chocolate consists of cocoa butter, cocoa solids, and sugar. The cocoa butter and cocoa solids come directly from the cacao bean, and the sum of their masses divided by the total mass of the chocolate results in the percentage advertised on the chocolate wrapper.
Chocolate’s ingredient list looks short and simple, especially before the common addition of milk, milk solids, or emulsifiers. However, its composition of fats is extremely complex.
Tempering Chocolate:
There are usually six or more types of cocoa butter in chocolate. Because of the varying structure and composition of these fats, they contain different textural properties, and solidify (crystalize) at different temperatures. Note that the crystallization temperature is also the melting temperature, since freezing and melting are both phase changes between solid and liquid states.
Each of the six crystal forms of cocoa butter, otherwise known as polymorphs, are listed below:
Polymorphs of Cocoa Butter
| Crystal Form | Crystalization Temperature (°F) | Chocolate Properties when Crystal is Present |
| I | 63 * | Crumbly and has evidence of “blooming”. This occurs when you put warm chocolate in the freezer and it rapidly cools. |
| II | 70 * | |
| III | 78 | Firm, but doesn’t snap. This occurs when chocolate is cooled in temperatures that are above freezing but are still low, like fridge cooling. |
| IV | 82 | |
| V | 94 | IDEAL! Glossy, snappy, and melts in your mouth |
| VI | 97 ** | Dense, hard, and doesn’t melt easily in your mouth. Requires months of sitting for formation of these crystals. |
*Because room temperature is around 70 °F, it is easy to see that polymorphs one and two would be less cohesive and more brittle.
** With the average temperature of humans being 98.6 °F and polymorph six’s crystallization temperature of ~97°F, there is not a large enough temperature difference for this solid to melt easily in the mouth.
To obtain the most optimal crystal form, V, without the less desirable ones, dark chocolate is first heated to around 120 °F to melt all crystals. It is then cooled to 80 °F to solidify forms of IV and above, and slowly heated to around 90 °F to melt the IV crystals. This leaves only the V crystals, since VI crystals take weeks of time to form and thus have not been created yet. When the V crystal form of chocolate cools, it has three coveted characteristics: shine, snap, and smoothness.
Milk and white chocolates have different tempering temperatures because of the addition of milk fats. See graph below for details.
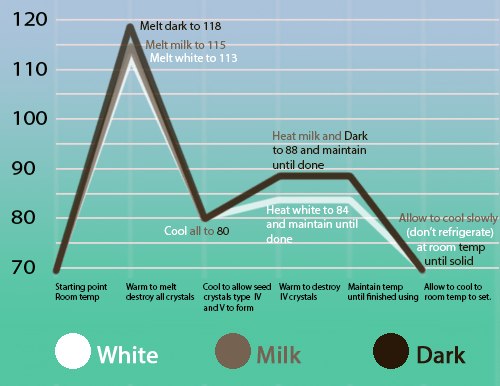
Temperatures are in degrees Fahrenheit. Image by Chocolate Alchemy.
Seizing:
If melted chocolate is contaminated with a drop of water, it seizes, becoming grainy and clumpy. This usually happens because tempered chocolate is an emulsion of polar cocoa and sucrose molecules in a non-polar base of fats. Water is polar, and therefore attracts the sugar and cocoa molecules, drawing them out of their immersion with the V crystal cocoa butter fats.
Funnily enough, a substantial amount of watery liquid won’t make chocolate seize, because it disintegrates the whole mixture and separates the molecules. This is one reason why adding cream to chocolate makes a smooth, viscous ganache. The additional fat also aids the process.
Sources: